
- Chemistry - Home
- Chemistry - Introduction
- Chemistry - Branches
- Chemistry - Radioactivity
- Chemistry - Nuclear Energy
- Chemistry - Metals
- Chemistry - Metallurgy
- Chemistry - Sodium
- Chemistry - Calcium
- Chemistry - Aluminum
- Chemistry - Magnesium
- Chemistry - Maganese
- Chemistry - Iron
- Chemistry - Copper
- Chemistry - Silver
- Chemistry - Gold
- Chemistry - Platinum
- Chemistry - Zinc
- Chemistry - Mercury
- Chemistry - Plutonium
- Chemistry - Uranium
- Chemistry - Lead
- Chemistry - Thorium
- Chemistry - Hydrogen
- Chemistry - Helium
- Chemistry - Oxygen
- Chemistry - Carbon
- Chemistry - Nitrogen
- Chemistry - Chemical Law
- Chemistry - Discovery of Elements
- Elements With Their Valence
- Elements With Their Atomic Number
- Chemistry - Nobel Prize
Chemistry Part 2 - Quick Guide
Chemistry - Introduction
Introduction
Chemistry is a branch of Natural Science that studies about the structure, composition, and changing properties of matters.
Chemistry studies the smallest part of a matter i.e. atom (along with its all properties) to the large materials (e.g. gold, silver, iron, etc.) and their properties.
Chemistry also studies the intermolecular forces (that provide matter the general properties) and the interactions between substances through the chemical reactions.

-
In 1998, Professor Raymond Chang defined Chemistry as −
"Chemistry" to mean the study of matter and the changes it undergoes.
It is believed that the study of chemistry started with the theory of four elements propounded by Aristotle.
The four theory of elements states that fire, air, earth, and water were the fundamental elements from which everything is formed as combination.
Because of his classical work namely The Sceptical Chymist, Robert Boyle, is known as the founding father of chemistry.
Boyle formulated a law, became popular as Boyles Law.
Boyles law is an experimental gas law that analyzes the relationship between the pressure of a gas and volume of the respective container.
By advocating his law, Boyle rejected the classical four elements theory.
The American scientists Linus Pauling and Gilbert N. Lewis collectively propounded the electronic theory of chemical bonds and molecular orbitals.
The United Nations declared 2011 as the International Year of Chemistry.
The matter is defined in chemistry as anything that has rest mass and volume and also takes space.
The matter is made up of particles.
The atom is the fundamental unit of chemistry.
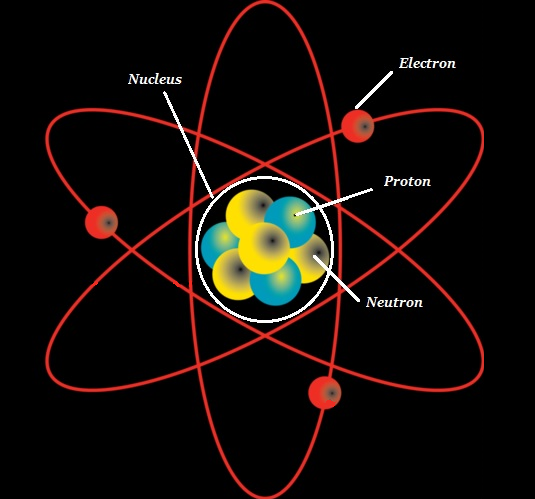
The atom consists of a dense core known as the atomic nucleus and it is surrounded by a space known as the electron cloud.
The nucleus (of an atom) is composed of protons (+ve charged particles) and neutrons (neutral or uncharged particles); collectively, these two are known as nucleons (as shown in the image given below).
A chemical element is a pure form of a substance; it consists of single type of atom.
The periodic table is the standardized representation of all the available chemical elements.
A compound is a pure form of a substance; it composed of more than one elements.
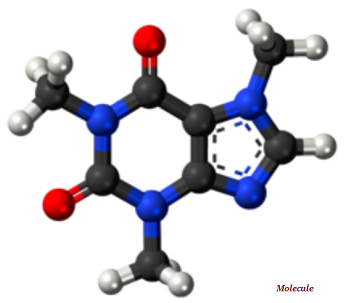
A molecule is the smallest indivisible part of a pure chemical substance; molecule has distinctive set of chemical properties (see the image given below).
Chemistry - Branches
The following table illustrates the branches of chemistry −
| Branch | Sub-branch | Definition |
|---|---|---|
| Physical Chemistry | Physical Chemistry | Study of the physical properties of molecules |
| Chemical Kinetics | Study of the rates of chemical reactions | |
| Electrochemistry | Study of the interaction of atoms, molecules, ions, and electric current (i.e. electron transfer between the electrode and the electrolyte or species) | |
| Surface chemistry | study of chemical reactions at surfaces (of substances) | |
| Thermochemistry | Study the relation between the chemical action and the amount of heat absorbed | |
| Quantum Chemistry | Study of application of quantum mechanics in physical models | |
| Spectroscopy | Study of spectra of light or radiation | |
| Photochemistry | study of the chemical effects caused by light | |
| Organic Chemistry | Organic Chemistry | Study of structure, properties, and preparation of the chemical (carbon) compounds (e.g. fuels, plastics, food additives, and drugs) |
| Stereochemistry | Study of the relative spatial arrangement of atoms (in molecules) | |
| Physical organic chemistry | study of structure and reactivity (interrelationship) in organic molecules | |
| Polymer Chemistry | Study of polymer molecules (composition and creation) | |
| Organometallic Chemistry | Study of chemicals that contain bonds (especially between a carbon and a metal) | |
| Medicinal chemistry | Study of designing, developing, and synthesizing the drugs & medicines | |
| Inorganic chemistry | Inorganic chemistry | Study of all materials that are not organic (such as minerals, metals, catalysts, crystal structures, etc.) |
| Organometallic Chemistry | Study of chemical compounds containing bonds (especially between carbon and metal) | |
| Solid-state Chemistry | Study of chemical compounds that contains bonds between carbon and metal | |
| Nuclear Chemistry | Study of radioactive substances | |
| Geochemistry | Study of chemical composition the earth (e.g. rocks, minerals & atmosphere) | |
| Bioinorganic Chemistry | Study of interactions between metal ions and living tissue | |
| Coordination Chemistry | ||
| Biochemistry | Biochemistry | Study of chemical reaction (and changes) in living beings |
| Molecular Biochemistry | Study of Biomolecules along with their functions | |
| Clinical Biochemistry | Study of chemical changes in living beings, caused by caused by different diseases | |
| Molecular Biology | Study of the different types of DNA, RNA, and protein biosynthesis (and their relationships) | |
| Agricultural biochemistry | Study of chemistry of fauna (i.e. plants) | |
| Analytical Chemistry | Study of standardized experimental methods in chemistry (i.e. quantitative determination of chemical properties of a substance) | |
| Astrochemistry | Study of the reactions of chemical elements and molecules found in the universe | |
| Cosmochemistry | Study of the chemical composition of the matters found in the universe | |
| Environmental chemistry | Study of chemical and biochemical phenomena that occur in the environment |
Chemistry - Radioactivity
Introduction
The process of emission of particles from nuclei because of the nuclear instability; is known as radioactivity.
The substance that releases such energy/rays is known as radioactive substance.
The invisible rays released from such radioactive substance are known as radioactive rays.
Likewise, radioactivity is a nuclear phenomenon that happens (naturally) because of the nuclear instability of atoms.
In 1896 Henri Becquerel first observed the phenomena of radioactivity, but the term radioactivity was coined by Marie Curie.
Marie Curie discovered the radioactive elements namely Polonium and Radium in 1898.
For her discovery, Marie Curie won the Nobel Prize.
Radioactive Rays
After long years of experiment, Ernest Rutherford along with his colleague (Hans Geiger and his student Ernest Marsden), discovered alpha rays, beta rays, and gamma rays.
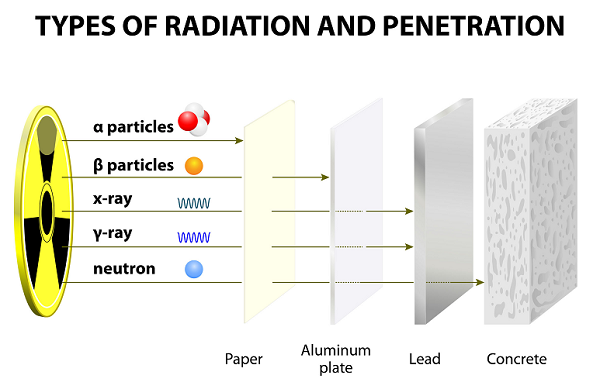
These rays emitted as the result of the disintegration of atoms.
Alpha () Particles
Alpha particles are usually composed of two protons and two neutrons, which are tightly bound together.
Alpha particles are being released during radioactive decay (or alpha decay) from the nucleus radio nuclides.
The alpha particles are identical to the nucleus of either normal helium atom or doubly ionized helium atom.
In comparison to other particles (i.e. Gamma and Beta), alpha particles are heavy and slow. Therefore, alpha particles have very small range in the air.
Because of slow speed, Alpha particles have very weak penetrating powers; these particles are even stopped by a thin paper sheet (see image given above).
Because of having the double positive charge, alpha particles are highly ionizing.
Beta () Particles
Beta particles are the fast moving electrons emitted by some radio nuclides during the radioactive decay (also known as beta decay).
Beta particles are of much lighter weight and carry a single negative charge.
Beta particles are rarely ionizing than the alpha particles.
Because of having lighter weight, beta particles can travel much farther than alpha particles; however, beta particles can be stopped by several sheet of papers or one sheet of aluminum.
Beta particles are negatively charged and get attracted towards positively charged particles.
Gamma () Particles
Gamma particles are the bundle of high energy namely electromagnetic energy (photon) emitted by the radioactive elements during the radioactive decay.
Among all three particles (alpha, beta, and gamma), gamma particles are the most energetic photons.
Gamma particles, which are the form of electromagnetic radiation(EMR), originate from the nucleus.
The wavelengths of gamma are the shortest among all three.
Gamma particles have no charge and they are neutral; therefore, they are unaffected by magnetic and electric fields.
Uses of Radioactive Elements
-
Radioactive elements are used in −
Medical field (treatment of many diseases)
Industrial process
Energy production Nuclear reactors
Chemistry - Nuclear Energy
Introduction
Nuclear reactions release tremendous amount of energy (known as nuclear energy), which are being used to produce electricity in a nuclear power plant.
The nuclear energy normally produced by nuclear fission, nuclear fusion, and nuclear decay.
In 1938, German chemists Otto Hahn, Fritz Strassmann, and the Austrian physicist Lise Meitner conducted the experiments in which the products of neutron-bombarded uranium. As result of this experiment, the relatively tiny neutron split the nucleus of the massive uranium atoms into two roughly equal pieces and released massive energy.
The nuclear experiments of Otto Hahn and his colleagues are popular as nuclear fission.
Nuclear Fission
The process of nuclear fission produces free neutrons and gamma photons, while doing this also releases a very large amount of energy.
Nuclear fission is an exothermic reaction, which can release large amounts of energy in the forms of electromagnetic radiation as well as kinetic energy.
Nuclear fission, sometimes, can occur naturally (i.e. without neutron bombardment) as a type of radioactive decay.
Types of Nuclear Fission
-
Following are the major types of Nuclear Fission −
Chain Reaction and
Fission Reaction
Lets discuss them in brief −
Chain Reaction
When one single nuclear reaction causes one or more subsequent nuclear reactions, it is known as chain reaction.
Such chain reaction increases the possibility of a self-propagating series of nuclear reactions.
The nuclear chain reactions release million times more energy per reaction than any other chemical reaction; therefore, it is also known as explosive or uncontrolled chain reaction.
When a heavy atom experiences nuclear fission, it normally breaks into two or more fission fragments. During the process, several free neutrons, gamma rays, and neutrinos are emitted, and ultimately a large amount of energy is released.
-
Following are the two examples of chain reaction −
235U + → neutron Fission fragments + 2.4 neutrons + 192.9 MeV
235Pu + → neutron Fission fragments + 2.9 neutrons + 198.9 MeV
In atom bomb, chain reaction technology is used, as it required consistent source of energy.
Fission Reactions
The fission reaction in which neutrons (produced by fission of fuel atoms) are used to induce yet more fission for the release of sustainable energy, is known as fission reactions.
Such reactions are slow and controllable; therefore, also known as controlled chain reaction.
The power (electricity) producing nuclear reactor is an ideal example of controlled chain reaction.
-
Based on the properties and type of usages, fission/controlled chain reaction is classified as −
Power reactors
Research reactors
Breeder reactors
These power reactors generally convert the kinetic energy of fission products into heat; further, the heat is used to heat a working fluid that drives a heat engine, which ultimately generates mechanical or electrical power.
Basic components of Nuclear Reactor
-
Following are the essential components of a nuclear reactor −
Nuclear fuels − Such as Uranium (233U, 235U), thorium (Th232), plutonium (Pu239).
Moderators − Used to control the emitted neutrons. E.g. heavy water, beryllium, graphite, etc.
Coolant − It is used to cool the reactor. E.g. water, steam, helium, CO2, air, molten metals, etc.
Control rods − It is used to run and stop the fission reaction. E.g. cadmium or boron rods are used for such purpose.
Nuclear Fusion
The process by which two light nuclei are fused to form a heavy nucleus is known as nuclear fusion; during this process, a tremendous amount of energy is being released known as nuclear energy.
The best example of nuclear fusion is hydrogen bomb.
A hydrogen bomb is about 1,000 times more powerful than an atom bomb.
Chemistry - Metals
Introduction
The material (which could be an element, compound, or alloy) that is characteristically hard, shiny, opaque, and has the property to conduct heat and electricity, is known as metal.
Metals are naturally found in the earths crust in impure form i.e. ores. And, it is extracted through mining process.
Among all known 118 elements (of the periodic table), about 91 elements are metals.

Features of Metals
-
Following are the significant features of metals −
Metals are generally malleable - it means, its shape can be changed permanently without breaking and cracking.
Metals are fusible it means; it can be fused or melted easily.
Metals are ductile it means; it can be given any shape even a thin sheet or wire.
Metals are good conductor of heat and electricity; heaver, lead is an exception, as it does not carry electricity.
Metals naturally react with various non-metals and forms compounds. Metals can react with bases and acids. E.g. 4 Na + O2 → 2 Na2O (sodium oxide), etc.
Alloys
An alloy is a product of the mixture of two or more elements in which metal dominates.
In order to produce or manufacture a desirable product, different metals (in different ratios) are mixed (i.e. alloys). E.g. alloys of iron namely stainless steel, cast iron, alloy sheet, etc. contribute a large proportion of both by quantity and commercial value.
Metals are usually made alloys with the purpose to make it more resistant to corrosion, less brittle, to give attractive colors, etc.
Metal Terminologies
Base Metal − In chemistry, the meaning of base metal is the metal that can be easily oxidized or corroded as well as reacts easily with HCl (dilute hydrochloric acid) and forms hydrogen. E.g. iron, nickel, zinc, lead, etc.
Ferrous Metal − "Ferrous" is a Latin word, which means the substance "containing iron." E.g. steel, etc.
Heavy Metal − The metal which are much denser than the normal metal is categorized as heavy metal. The heavy metals are toxic or poisonous at low concentrations. E.g. mercury (Hg), arsenic (As), chromium (Cr), cadmium (Cd), thallium (Tl), and lead (Pb).
Precious Metal − The metallic elements, which have rare metallic chemical element of high economic value, is categorized as precious metal. E.g. platinum, gold, silver, palladium, etc.
Noble Metal − The metals that are resistant to corrosion or oxidation. E.g. ruthenium (Ru), rhodium (Rh), palladium (Pd), etc.
Application of Metals
-
Following are the significant applications of the metals −
As metals are good conductor of heat and electricity; therefore, it is used as electric wire and in many other electric appliances including electric motors, etc. E.g. copper, silver, aluminum, etc.
Heavy metals are being used in the constructions of bridge, pool, and for many such purposes.
Many metals are used to manufacture various home items, such as, utensils, pots, stoves, etc.
Metals are frequently used to manufacture many types of tools ranging from a simple screw driver to a heavy rod roller.
Precious metals have beautiful look and they are attractive (e.g. gold, silver, etc.); therefore, they are used as ornaments.
Some specific metal is used for heat sinks that protects the sensitive equipment from overheating.
Radioactive metals (e.g. uranium and plutonium) are used in the generation of nuclear energy.
Mercury is a metal that remains in liquid form at room temperature; it is used in thermometer.
Chemistry - Metallurgy
The branch of science and technology that studies the properties of metals and their production and purification is known as metallurgy.
Naturally occurring solid inorganic substance is known as a mineral.
Naturally occurring solid material from which valuable mineral or metal can be extracted is known as ore.
The following table illustrates the major elements and their ores −
| Element | Ores | Chemical Composition |
|---|---|---|
| Aluminum | Bauxite | Al2O32H2O |
| Corundum | Al2O3 | |
| Kryolite | Na3AlF6 | |
| Dyspore | Al2O3.H2O | |
| Copper | Copper Pyrite | CuFeS2 |
| Malachite | 2CuCO3Cu(OH)2 | |
| Iron | Hematite | Fe2O3 |
| Magnetite | Fe3O4 | |
| Siderite | FeCO3 | |
| Sodium | Sodium Carbonate | Na2CO3 |
| Sodium Chloride | NaCl | |
| Sodium Nitrate | NaNO3 | |
| Sodium Sulphate | Na2SO4 | |
| Potassium | Potassium Chloride | KCl |
| Potassium Carbonate | K2CO3 | |
| Potassium Nitrate | KNO3 | |
| Magnesium | Magnesite | MgCO3 |
| Dolomite | CaMg(CO3)2 | |
| Epsom Salt | MgSO4 | |
| Calcium | Calcium Carbonate | CaCO3 |
| Tin | Cassiterite | SnO2 |
| Lead | Galena | PbS |
| Cerussite | PbCO3 | |
| Anglesite | PbSO4 | |
| Silver | Argentite | Ag2S |
| Tetrahedrite | Sb4S3 | |
| Zinc | Zinc Carbonate (known as calamine) | ZnCO3 |
| Zinc Sulphide | ZnS | |
| Mercury | Cinnabar | HgS |
| Manganese | Pyrolusite | MnO2 |
| Phosphorous | Phosphorite | Ca3(PO4)2 |
| Fluorapatite | Ca5 (PO4)3F | |
| Chlorapatite | 3Ca3(PO4)2.CaCI2 | |
| Gold | Calaverite | AuTe2 |
| Sylvanite | (Ag,Au)Te2 | |
| Nagygite | (Pb5Au(Te, Sb)4S5-8) | |
| Petzite | Ag3AuTe2 | |
| Antimony | Stibnite | Sb2S3 |
| Stibiconite | (Sb3+ Sb25+ O6(OH)) | |
| Cobalt | Cobaltite | CoAsS |
| Nickel | Pentlandite | ((Ni, Fe)S) |
| Chromium | Chromite | (FeCr2O4) |
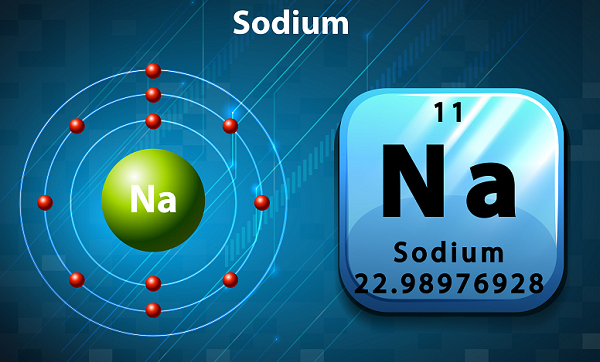
Chemistry - Sodium
Introduction
Sodium is a soft, silvery color, and highly reactive alkali metal.
In the periodic table, Sodium is kept in group 1, as it has single electron in its outer shell.
The symbol of sodium is Na, which has been actually taken from Latin word natrium.
In terms of abundance, sodium is the sixth element found in the Earth's crust.
Sodium exists in various minerals including feldspars, sodalite, and rock salt (NaCl).
In 1807, Humphry Davy first isolated sodium by the electrolysis of sodium hydroxide.
By the time, 20 isotopes of sodium are known, but among all, only 23Na is stable.
Salient Features of Sodium
-
Following are the major features of sodium element −
Sodium metal a soft element that be can be easily cut with a knife.
Sodium is a good conductor of heat and electricity.
Because of having low atomic mass and large atomic radius, sodium is one of the least dense elements (third least dense element first two are lithium and potassium).
Sodium can float on water.
Sodium along its compounds glow yellow (see image below).

Sodium compounds have very high commercial importance and have high demand in the industries of glass, paper, soap, and textiles.
Sodium Compounds
-
Following are some of the significant examples of sodium compounds −
Table salt -(NaCl)
Soda ash -(Na2CO3)
Baking soda -(NaHCO3)
Caustic soda -(NaOH)
Sodium nitrate -(NaNO3)
Sodium thiosulfate -(Na2S2O35H2O)
Borax -(Na2B4O710H2O)
Occurrence of Sodium
The crust of Earth contains about 2.27% sodium.
Sodium is the 5th most abundant metal; other four are aluminum, iron, calcium, and magnesium.
In the oceanic water, about 1.08 × 104 milligrams sodium found in per liter.
Sodium does not found as a pure element, as it is highly reactive.
Uses of Sodium
-
Following are the major uses of sodium −
Sodium chloride is highly useful for anti-icing and de-icing as well as a preservative.
In cooking, sodium bicarbonate is used.
Sodium and some of its compounds are used in medicines.
In comparison to potassium (which is a better ion), sodium is more frequently used because of its lower price and atomic weight.
In organic chemistry, sodium hydride is used as various reactions.
Metallic sodium is principally used for the production of sodium borohydride, sodium triphenylphosphine, azide, indigo, etc.
In some fast reactors, liquid sodium is used as a heat transfer fluid because of having the property of good heat conductivity.
Sodium is also an essential mineral for the human health, as it regulates blood pressure, blood volume, osmotic equilibrium, and pH value.
The minimum amount of 500 milligrams sodium is required every day for a healthy human body.
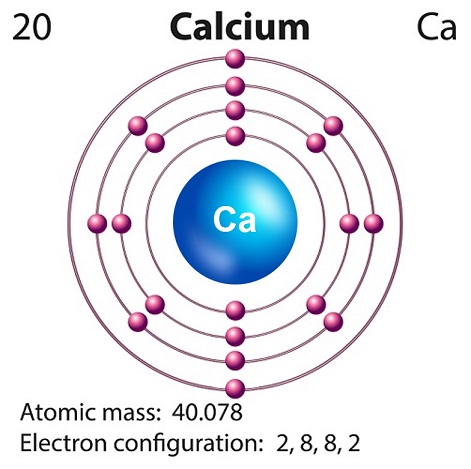
Chemistry - Calcium
Introduction
Calcium is a soft silvery-white alkaline element found largely in the Earths crust.
Calciums symbol is Ca and atomic number is 20.
Free calcium hardly exists in nature, as it is highly reactive.
Calcium is produced usually in supernova nucleosynthesis.
Salient Features of Calcium
Calcium is one of the most abundant metals by mass found in many animals.
Calcium is a very important constituent of teeth, bone, and shells.
Calcium carbonate and calcium citrate are the major dietary supplements required for a good health.
World Health Organization (WHO) listed calcium as the Essential Medicines.
In comparison to other metals, the calcium ion and most of the other calcium compounds have low toxicity.
If calcium comes in contact with water or acid, it reacts with them and becomes hazardous.
If calcium comes in contact with air, it reacts swiftly and forms a gray-white coating of calcium nitride and calcium oxide.
Most of the calcium salts are normally colorless.
When calcium burnt, the color of its flame appears brick red (see the image given below).

Calcium metal has comparatively a higher electrical resistivity than aluminum or copper.
Occurrence
Calcium occurs usually in sedimentary rocks.
The minerals (sedimentary) in which calcium found are calcite, dolomite, and gypsum.
Calcium also found in igneous and metamorphic rocks mostly in the silicate minerals, such as amphiboles, plagioclases, pyroxenes, and garnets.
Calcium also found in many of the food products namely dairy products, almonds, hazelnuts, soy beans, broccoli, dandelion leaves, figs, and in many more.
Compounds of Calcium
Calcium oxide - CaO
Calcium hydroxide - Ca(OH)2
Calcium chloride - CaCl2
Calcium hypochlorite (Bleaching powder) - Ca(ClO)2
Calcium phosphate - Ca3(PO4)2
Uses of Calcium
-
Calcium has wide range of usage, significant of them are −
Calcium carbonate (CaCO3) is used in manufacturing cement.
Calcium carbonate (CaCO3) is also used in making toothpaste.
In insecticides, calcium arsenate (Ca3(AsO4)2) is used.
Calcium chloride (CaCl2) is used in ice removal as well as in dust control.
Calcium citrate (Ca3(C6H5O7)2) is commonly used as food preservative.
Calcium gluconate (Ca(C6H11O7)2) is used frequently as a food additive as well as in vitamin pills.
Calcium hypochlorite (Ca(OCl)2) is generally used as a swimming pool disinfectant, as a bleaching agent.
Chemistry - Aluminum
Introduction
The metal with color silvery-white, soft, nonmagnetic, and ductile metal property, is known as aluminum.
The symbol of aluminum is Al and its atomic number is 13.
The Chemical element aluminum belongs to the boron group.

- Bauxite is the chief ore of the aluminum.
Salient Features of Aluminum
Aluminum metal is a chemically reactive element.
Aluminum has the potential to resist corrosion and the process of this resistivity is known as passivation.
Aluminum is a comparatively durable, lightweight, soft, malleable, and ductile, metal.
Aluminum is nonmagnetic and does not get ignite easily.
An aluminum film is a very good reflector of visible light, as it reflects more than 90 percent of incoming rays.
Aluminum commonly reacts with water and forms hydrogen.
Aluminum is the metal of low density and it has the property to resist corrosion.
Aluminum has the property of heat and electricity conductivity and hence, it is a good conductor.
Occurrence of Aluminum
Aluminum makes up (about) 8 percent of the Earth's crust.
After oxygen and silicon, aluminum is the third most abundant element; however, it is the most abundant metal in the crust.
When hydrogen fuses with magnesium, it creates stable aluminum.
Aluminum naturally found in oxides or silicates states.
Compounds of Aluminum
-
Following are the major compounds of aluminum −
Alumina - Al2O3
Aluminum chloride - AlCl3
Aluminum sulphate - Al2(SO4)3
Aluminum hydroxide - Al(OH)3
Aluminum carbide - Al4C3
Usage of Aluminum
-
Aluminum has wide range of usages in the industries as well as in everyday life; significant of them are −
Aluminum is used in transportation industries, such as railway, automobiles, aircraft, spacecraft, trucks, marine vessels, bicycles, etc.
Aluminum is used in packaging some specific materials.
Aluminum is used in the constructions of doors, windows, building wire, sheathing, roofing, etc.
Aluminum is largely used in making electric wire.
Aluminum is used in making home appliances and many other household items, such as cooking utensils.
Aluminum is used in baseball bats, watches, and many more such kind of stuffs.
Aluminum is used in photographic equipment.
Aluminum is used in electronic appliances.
Aluminum is used as light reflector, as it is a good light reflector; basically, some of the materials are aluminum coated specially to reflect light.
Aluminum is used in production of hydrogen gas by reaction with hydrochloric acid.
Aluminum is used in manufacturing musical instruments.
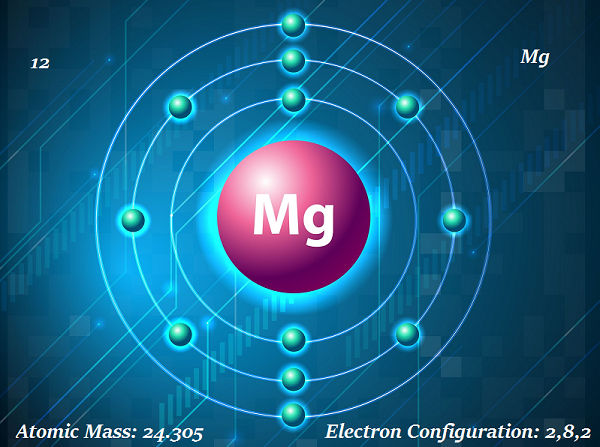
Chemistry - Magnesium
Introduction
Magnesium is a shiny gray solid element.
The symbol of magnesium is Mg and atomic number is 12.
With approximately 80% of the world market share, China is the largest supplier of magnesium.
Salient Features of Magnesium
The density of magnesium is two-thirds the density of aluminum.
Among all the alkali metals of the Earth, magnesium has lowest melting point (i.e. about 1,2020F) and lowest boiling point (about 1,9940F).
Magnesium usually reacts with water at room temperature.
Sometimes, magnesium is also used as an igniter for thermite.
Magnesium, when burns in air, produces a brilliant-white light, which also includes strong ultraviolet wavelengths.

Magnesium, when burns, it produces intense bright and white light (see image given above).
Occurrence of Magnesium
By mass, magnesium is the eighth-most-abundant element found in the Earth's crust.
Magnesium is found usually in large deposits of magnesite, dolomite, and other such minerals.
The soluble magnesium ion is found in the mineral water.
After sodium and chlorine, magnesium is the third most abundant element dissolved in seawater.
Magnesium naturally occurs only in combination with some other elements.
By mass, magnesium is the 11th most abundant element in the human body and it is essential to all cells and enzymes.
Magnesium ions frequently interact with polyphosphate compounds including ATP, DNA, and RNA.
Compounds of Magnesium
-
Following are the major compounds of magnesium −
Magnesium carbonate - MgCO3
Magnesium chloride - MgCl2
Magnesium citrate - C6H6MgO7
Magnesium hydroxide - Mg(OH)2
Magnesium oxide - MgO
Magnesium sulfate - MgSO4
Magnesium sulfate heptahydrate - (MgSO47H2O)
Magnesium sulfate heptahydrate is commonly known as Epsom salt.
Usages of Magnesium
-
Magnesium has wide range of usage in our lives; however, some significant usages of magnesium are −
After iron and aluminum, magnesium is third most commonly used element.
Magnesium is especially used in super-strong, lightweight materials, and alloys.
Magnesium is also used as engine materials in the aircraft industry.
Magnesium is also used to purify the solvents; such as in preparing the super-dry ethanol.
Many of the automotive big brands including Mercedes, Porsche, BMW, Volkswagen, Chevrolet, etc. use magnesium in making their highly quality cars.
Because of having low weight and good electrical and mechanical properties, magnesium is commonly used in manufacturing laptops and tablet computers, mobile phones, cameras, and many other electronic components.
Magnesium sulfite is usually used in manufacturing paper.
Chemistry - Maganese
Introduction
Manganese is a chemical element that usually found in combination with the iron.
The symbol of manganese is Mn and atomic number is 25.
Manganese is a metal very important for the industrial use.

In 1774, Johan Gottlieb Gahn, first time isolated an impure sample of manganese metal in 1774.
Features of Manganese
-
Following are the major features and characteristics of manganese −
Similar to iron, manganese is silvery-gray metal.
Manganese can be oxidized easily, but very difficult to fuse it, as it is very hard and brittle.
In air, manganese gets tarnished slowly (oxidization).
Manganese is an element, which is part of the iron group.
Occurrence of Manganese
Manganese is the 12th most abundant element of the earths crust.
Soil usually contains about 79000 ppm of manganese with an average of 440 ppm.
Seawater has only about 10 ppm manganese; whereas, the atmosphere contains about 0.01 g/m3.
Pyrolusite (MnO2) is the most important ore of manganese.
Compounds of Manganese
-
Following are the major compounds of manganese −
Manganese (II) oxide - MnO
Manganese (I) oxide - Mn2O3
Manganese dioxide - MnO2
Manganese chloride - MnCl2
Potassium permanganate - KMnO4
Manganese (II) sulfate - MnSO4
Manganese (II) carbonate - MnCO3
Manganese (II) sulfide - MnS
Manganese (II) nitrate - Mn(NO3)2
Manganese (II) bromide - MnBr2
Manganese heptoxide - Mn2O7
Dimanganese decacarbonyl - C10O10Mn2
Manganese (II) iodide - MnI2
Manganese (II) fluoride - MnF2
Uses of Manganese
-
Following are the major uses of manganese −
Manganese is one of the most essential constituents of steel production.
Manganese phosphating is commonly used for the rust and corrosion prevention on steel.
In biology, manganese(II) ions act as cofactors for the large variety of enzymes.
Manganese is also important in the oxygen-evolving phenomenon of photosynthetic plants.
Manganese dioxide is also used in the manufacture of oxygen and chlorine and in drying black paints.
Chemistry - Iron
Introduction
Iron is the most common element found in largely in outer as well inner core of the earth.
The symbol of iron is Fe and atomic number is 26.

Iron is one of the earliest known elements that is being used by human beings.
Salient Features of Iron
-
Following are the major features of iron −
Pure iron element is soft, ductile, and malleable.
The boiling point of iron ranges between 15330C and 24500C.
Iron easily gets attracted towards magnet.
In dry air, iron remains inactive and does not react (with air); however, in moist air, it reacts and forms rust.
Pure iron normally does not react with pure water; however, it reacts easily with ordinary of polluted water and rust forms.
Iron reacts with halogen and Sulphur to form halide and sulphide accordingly.
Occurrence of Iron
The inner and outer cores of the earth are largely made up of iron and nickel.
Most likely, iron is the most abundantly available element of the earth; however, it is the fourth most abundantly available element of the crust.
Types of Iron
-
Following are the major types iron −
Hematite - Fe2O3
Magnetite - Fe3O4
Siderite - FeCO3
Compounds of Iron
-
Following are the major compounds of iron −
Iron (II) oxide - FeO
Iron (III) chloride - FeCl3
Iron (III) oxide-hydroxide - Fe(OH)3
Iron (II) sulfide - FeS
Iron (II) chloride - FeCl2
Iron phosphate - FePO4
Ferrate (VI) - (FeO4)2-
Iron (II) acetate - Fe(C2H3O2)2
Iron (III) sulfide - Fe2S3
Iron (III) chromate - Fe2(CrO4)3
Iron (II) hydroxide - Fe(OH)2
Iron (III) acetate - C14H27Fe3O
Iron (II) oxalate - FeC2O4
Iron (II) fluride - FeF2
Uses of Iron
Among all the metals, iron is most widely used (about 90 percent of worlds total metal production).
In most of the heavy industries, iron is the most essential element.
Industries like railway, ship building, automobile, engineering construction, etc., everywhere, iron is essentially required.
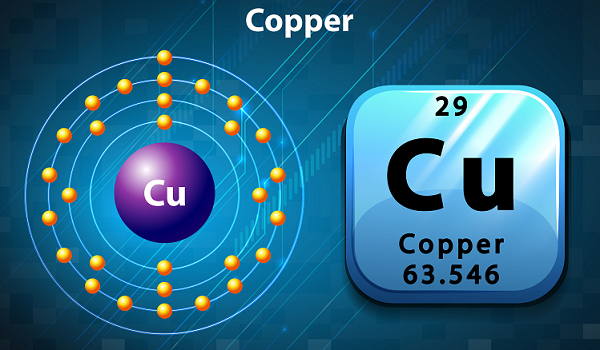
Chemistry - Copper
Introduction
Copper is a soft, ductile, and malleable metal.
Copper has very high thermal and electrical conductivity.
The symbol of copper is Cu and atomic number is 29.
Copper is known to people since (about) c. 8000 BC.
Copper is the first metal, which was smelted from its ore around c. 5000 BC.
Salient Features of Copper
Copper was the first metal that cast into a shape (in a mold).
Copper was the first metal that successful alloyed with another metal, e.g. copper alloyed with tin and resultantly bronze is prepared. It is done about c. 3500 BC.
First metal to be purposefully alloyed with another metal, tin, to create bronze, c. 3500 BC.
Copper has a natural reddish-orange color; it is visible once the its surface is exposed.
Copper is a very good conductor of electricity and heat.
Copper is an element of group 11 of the periodic table.
Copper normally does not react with water; however, it does react slowly with atmospheric oxygen and form a layer of brown-black copper oxide.
The brown-black copper oxide usually protects the underlying metal from further corrosion just like passivation.
Passivation is a process of use of a light coat of a protective material, such as metal oxide, which is used to create a protective shell against the corrosion.
Occurrence of Copper
Copper is commonly found in the earths crust.
In 1857, the largest mass of elemental copper (weighing about 420 tones) discovered. It was found on the Keweenaw Peninsula located in Michigan, US.
Alloys of Copper
The metal alloys, which have the copper as their major constituent, is known as copper alloys.
Copper alloys are highly resistant to corrosion.
The best (traditional) example of copper alloy is bronze (made by mixing tin and brass).
-
Following are the major alloys of copper −
Brass
Bronze
Auricupride
Chinese silver
Corinthian bronze
Electrum, Green gold
Grey gold
Niello
Panchaloha
Rose, red, and pink gold
Spangold
Shibuichi
Tibetan silver
White gold
Compounds of Copper
-
Following are the major compounds of the copper −
Cupric acetate - Cu(CH3COO)2
Copper(I) oxide - Cu2O
Copper(II) oxide - CuO
Copper(II) chloride - CuCl2
Dicopper chloride trihydroxide - Cu2(OH)3Cl
Copper(I) chloride - CuCl
Copper(II) nitrate - Cu(NO3)2
Copper Cyanide - CuCN
Uses of Copper
Copper is largely used in making electric wires.
Copper is used in electric motors.
Copper is used in roofing, plumbing, and in many other industries.
Copper paint is used in painting boats and many other materials.
Many of the home appliances are made up of either pure copper or its alloys.
Chemistry - Silver
Introduction
Silver is a soft, lustrous transition, and white metal.
Silver has the highest electrical and thermal conductivity; and, it has also the highest reflectivity of any metal.

The symbol of silver is Ag and atomic number is 47.
Salient Features of Silver
Silver is a precious metal used since long time by human beings.
Silver is an element of group 11 of the periodic table.
Silver has an excellent white metallic luster commonly used in a high polish.
As silver has no color; therefore, it has high reflectivity (of light).
Silver has very high electrical and thermal conductivity. Its electric conductivity is the highest higher than copper.
Among all the metals, silver also has the lowest contact resistance.
Occurrence of Silver
The metal silver is usually found in the Earth's crust in the pure form.
Silver also found as an alloy with the gold and some other metals.
Silver is also found in some minerals, such as argentite and chlorargyrite.
Silver is largely produced as a byproduct of gold, copper, zinc, lead, etc.
Alloys of Silver
-
Following are the major alloys of silver −
Argentium sterling silver
Britannia silver
Dor bullion
Electrum
Goloid
Platinum sterling
Sterling silver
Tibetan silver
Compounds of Silver
-
Following are the major compound of silver −
Silver chloride - AgCl
Silver iodide - Agl
Silver bromide - AgBr
Silver oxide - Ag2O
Silver sulfide - Ag2S
Silver fluoride - AgF
Silver cyanide - AgCN
Silver carbonate - Ag2CO3
Silver acetate - AgC2H3O2
Silver sulfate - Ag2SO4
Silver chromate - Ag2CrO4
Silver oxalate - Ag2C2O4
Silver chlorate - AgClO3
Uses of Silver
-
Following are the major uses of silver −
From the ancient period, silver is being used in making coins.
Silver is also used in making ornaments.
Many of the home pots and other utensils were also used to be made by silver.
Silver has also medicinal use, as it is used as an antibiotic coating in medical devices.
Because of having very high electric conductivity, silver is commonly used in some electronic devices.
Besides, silver has many other uses, such as in photography, in chemical equipment, nanoparticles, etc.
Chemistry - Gold
Introduction
Gold is a bright, reddish yellow, soft, dense, malleable, and ductile metal naturally found in the earths crust.
The symbol of gold is Au and atomic number is 79.

Gold is (chemically) a transition metal and belongs to group 11 of the periodic table.
Salient Features of Gold
Gold, which remains in a solid state under standard conditions, is the least reactive element.
Gold is resistant to most of the acids.
Gold does dissolve in aqua regia; aqua regia is a mixture of nitric acid and hydrochloric acid.
However, gold is insoluble in nitric acid.
Gold usually dissolves in alkaline solutions of cyanide.
Cyanide solutions are commonly used in mining and electroplating.
Gold also dissolves in mercury and forms amalgam alloys.
Gold does not react with oxygen at any temperature.
Occurrence of Gold
Gold commonly occurs as a free element i.e. in the natural form.
Gold occurs as nuggets or else found in in rocks, grains, in veins, and in some other alluvial deposits.
Gold also occurs in a solid solution forms with the native element such as silver (as electrum).
At some places, gold also naturally alloyed with copper and palladium.
Alloys of Gold
-
Following are the major alloys of gold −
Colored gold
Crown gold
Electrum
Rose gold
Tumbaga
White gold
Compounds of Gold
-
Following are the major compounds of the gold −
Gold (III) chloride - AuCl3
Gold (I) chloride - AuCl
Cyanide - CN
Chloroauric acid - HAuCl4
Gold (III) oxide - Au2O3
Gold bromide - AuBr
Aqua regia - HNO3+3HCl
Gold bromide - AuBr3
Gold (III) hydroxide - AuH3O3
Gold fluoride - AuF3
Gold (V) fluoride - AuF5
Gold sulfide - Au2S
Fulminating gold
Gold salts
Gold heptafluoride
Uses of Gold
Gold is one of the oldest elements that human being have been using for thousands of years.
As it is highly precious and provides a beautiful look, hence it is characteristically used in making ornaments.
As per the recent trend (of the world), about 50% gold is used in making jewelry, 40% used in investments, and remaining 10% is used in industry.
Chemistry - Platinum
Introduction
Platinum is a malleable, ductile, dense, and highly unreactive chemical element.
The symbol of platinum is Pt and its atomic number is 78.

The name of platinum is derived from a Spanish term i.e. platina, which means "little silver."
Platinum is the most precious and the rarest metal (element) on the earth.
Salient Features of Platinum
-
Following are the important features of the platinum −
Platinum is a silver white metal.
Platinum is an element of group 10 of the periodic table.
Platinum is one of the rarest elements in the crust of the earth.
Platinum is one of the least reactive elements.
Platinum has six natural isotopes.
Platinum is one of the most highly valuable and precious metals.
Platinum is characteristically resists corrosion in all conditions. Because of this reason, it is considered as noble metal.
Platinum is normally insoluble in nitric and hydrochloric acid, but dissolves in hot aqua regia.
After dissolving into the hot aqua regia, platinum gives aqueous chloroplatinic acid (see the image given below).

Occurrence of Platinum
Platinum is commonly found as the native (natural) platinum and as alloy with the other platinum-group.
Platinum usually occurs in the ores of nickel and copper.
Platinum also occurs naturally in the alluvial sands (commonly found in rivers).
Platinum occurs with the concentration of only (about) 0.005 ppm in the Earth's crust.
Alloys of Platinum
Platinum-iridium is one of the most significant alloys of platinum.
Compounds of Platinum
-
Following are the major compounds of platinum −
Platinum (II) chloride - Pt Cl2
Platinum (IV) chloride - PtCl4
Adams catalyst - PtO2
Platinum hexafluoride - PtF6
Potassium tetrachloropla - K2PtCl4
Krogmanns salt - K2Pt(CN)4Br
Chloroplatinic acid - H2PtCl6
Sodium hexachloropl - Na2PtCl6
Aqua regia - HNO3+3HCl
Uses of Platinum
Platinum is used largely for vehicle emissions control devices.
Platinum is used in petroleum refining and many other chemical productions.
Platinum is used in electronic devices, such as in hard disk (drives).
Platinum is also used in jewelry.
Apart from all these usages, platinum is also used in medicine (anti-cancer drugs), glassmaking equipment, electrodes, turbine engines, investment, etc.
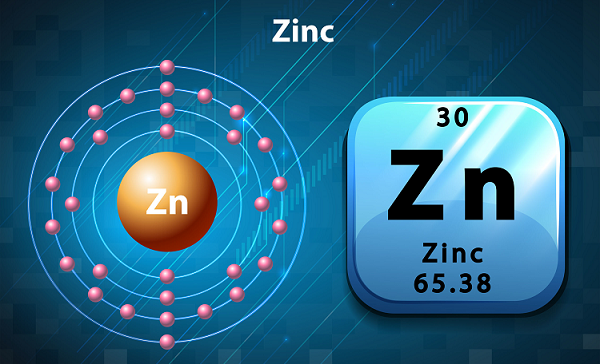
Chemistry - Zinc
Introduction
In the periodic table, zinc is the first element of group 12.
The symbol of zinc is Zn and the atomic number is 30.
In terms of availability, zinc is the 24th most abundant element found in the Earth's crust and it has five stable isotopes.
The most common zinc ore is sphalerite (zinc blende), which is a zinc sulfide mineral.
Andreas Sigismund Marggraf, the German chemist, first discovered the pure metallic zinc in 1746.
Interestingly, alchemists burned zinc in the air and form something different thing and they named that "philosopher's wool" or "white snow."
Salient Features of Zinc
Zincs color is bluish-white and it is lustrous and diamagnetic metal.
Zinc metal is normally hard and brittle; however, at when the temperature increases from 1000C, it becomes malleable.
When temperature increases 2100C, then the zinc metal again becomes brittle and can be pulverized easily by beating.
Zinc is a conductor of electricity.
Occurrence of Zinc
Zinc usually found in association with some other base metals such as copper and lead.
Sphalerite is a form of zinc sulfide and it is the most heavily mined ore.
Sphalerite contains about 60 to 62% zinc.
Alloys of Zinc
-
Following are the major alloys of zinc −
Brass
Nickel silver
German silver
Compounds of Zinc
-
Following are the major compounds of zinc −
Zinc oxide - ZnO
Zinc sulfide - ZnS
Zinc halides - ZnF2
Zinc nitrate - Zn(NO3)2
Zinc chlorate - Zn(ClO3)2
Zinc sulfate - ZnSO4
Zinc phosphate - Zn3(PO4)2
Zinc molybdate - ZnMoO4
Zinc chromate - ZnCrO4
Zinc arsenite - Zn(AsO2)2
Zinc acetate - Zn(O2CCH3)2
Uses of Zinc
Zinc is one of the most important elements for the public health.
Zinc is largely used as an anti-corrosion agent and coating of iron and steel materials.
Zinc is commonly used as the anode or fuel of the zinc-air battery.
Zinc oxide is extensively used as a white pigment (see the image given below) in paints.

Zinc oxide is also used as a catalyst in manufacturing rubber.
Zinc is an essentially required element for our health; it is normally used as supplementary materials in the forms as zinc oxide, zinc acetate, or zinc gluconate.
Zinc is normally antioxidant material.
Zinc deficiency in human body may cause major depressive disorder.
After the bodily injury, zinc is used to speed up the healing process.
Zinc pyrithione is commonly used in shampoos to prevent the dandruff.
Chelated zinc is usually used in toothpastes and mouthwashes (liquid), as it prevents the bad breath.
Zinc also protects skin from sunburn, therefore, it is used in body lotions.
Chemistry - Mercury
Introduction
Mercury is a chemical element, which usually known as quicksilver.
Formerly, mercury was named as hydrargyrum.
The symbol of mercury is Hg and atomic number is 80.

The red pigment vermilion is extracted by grinding either natural cinnabar or synthetic mercuric sulfide.
Salient Features of Mercury
Mercury is a heavy and silvery-white metal.
Mercury usually available in liquid state; in normal condition, it is only the few metallic element that remains in liquid state at room temperature.
Mercury is the poor conductor of heat, but it is a good conductor of the electricity.
The freezing point of mercury is 38.830C and the boiling point is 356.730C.
Mercury poisoning caused because of the ingesting any form of mercury. It is also caused by inhalation of mercury vapor.
Mercury dissolves many metals including gold and silver to form amalgams.
Occurrence of Mercury
Mercury is one of the rarest element in the earths crust.
The richest mercury ores carry about 2.5 percent mercury (in terms of mass).
Mercury is found either as a native (natural) element) or in corderoite, cinnabar, livingstonite, etc. minerals.
Mercury is found the region of young mountain belt; the belt that keep forcing the denser rocks to the crust of the earth. For example, volcanic region or even hot spring regions.
Alloys of Mercury
Amalgam is the major alloy of mercury.
Compounds of Mercury
-
Following are the major compounds of Mercury −
Mercury (II) chloride - HgCl2
Mercury (II) oxide - HgO
Mercury sulfide - HgS
Mercury (I) chloride - Hg2Cl2
Dimethylmer - C2H6Hg
Mercury (II) nitrate - Hg(NO3)2
Mercury (II) acetate - C4H6O4Hg
Mercury (II) sulfate - HgSO4
Mercury (I) iodide - Hg2I2
Mercury selenide - HgSe
Mercury (II) fulminate - Hg(CNO)2
Mercury (II) bromide - HgBr2
Mercury (II) iodide - HgI2
Mercury (I) oxide - Hg2O
Mercury (I) fluoride - Hg2F2
Mercuric amidochloride - ClH2HgN
Diethylmercury - C4H10Hg
Uses of Mercury
Mercury is largely used in chemical industries.
Mercury is used in electrical and electronic application.
Mercury is used in the thermometers that we use to measure the temperature.
Mercury along with its compounds are commonly used in various medicines.
Chemistry - Plutonium
Introduction
Plutonium is basically an actinide metal and it appears like silvery-gray.
The element, which atomic number is ranging between 89 and 103, is known as actinide element.
The symbol of plutonium is Pu and atomic number is 94.

Plutonium normally possesses six allotropes.
Plutonium is named after Pluto.
Plutonium was first discovered in 1940, by a group of scientists namely Glenn T. Seaborg, Joseph W. Kennedy, Edwin M. McMillan and Arthur C. Wahl.
Salient Features of Plutonium
Plutonium is a radioactive chemical element.
Plutonium when exposed to air, it gets tarnished and when it oxidized, it forms a dull coating.
Plutonium reacts with many elements including halogens, nitrogen, carbon, silicon, and hydrogen.
Because of fission process, neutrons get released and convert uranium-238 nuclei into plutonium-239.
Plutonium-239 and plutonium-241 both are fissile, and hence, they can sustain a nuclear chain reaction. It is very well applicable in nuclear weapons and nuclear reactors.
The melting point of plutonium is 640 0C and its boiling point is 3,228 0C.
The release of helium nucleus (a high-energy) is the most common form of radioactive decay for the plutonium.
Occurrence of Plutonium
The plutonium naturally found only in trace amounts within the uranium deposits.
Plutonium is also extracted by burning the uranium (while developing nuclear energy).
Alloys of Plutonium
-
Following are the major alloys of plutonium −
Plutoniumgallium is one of the important alloys of plutonium as well as of gallium.
Plutoniumgallium is used in the nuclear weapon pits.
Plutoniumgallium has the property of very low thermal expansion.
-
Some other alloys of plutonium are −
Plutoniumaluminum
Plutoniumgalliumcobalt
Plutoniumzirconium
Plutoniumcerium
Plutoniumuranium
Plutoniumuraniumtitanium
Thoriumuraniumplutonium
Compounds of Plutonium
Plutonium (IV) oxide - PuO2
Plutonium (III) chloride - PuCl3
Plutonium tetrafluoride - PuF4
Uses of Plutonium
The isotope plutonium-239 is one of the significant elements in developing the nuclear weapons.
Plutonium is used as a fuel in the nuclear power plants.
Chemistry - Uranium
Introduction
Uranium is the metal of the actinide series of the periodic table.
The symbol of uranium is U and atomic number is 92.
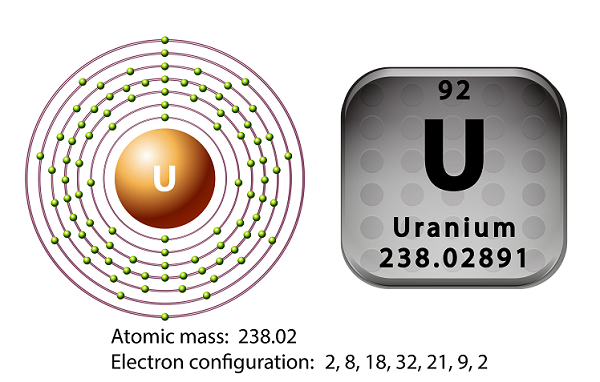
In 1789, Martin Heinrich Klaproth had discovered the element uranium and named it after the name of Uranus.
Salient Features of Uranium
Uranium is a silvery-white metal.
A uranium atom has 92 electrons as well as 92 protons, of which 6 are valence electrons.
Because of having unstable isotopes, uranium is a weak radioactive element.
Uranium-238 is the most common isotope of uranium.
Uranium occurs naturally in very low concentrations i.e. a few parts per million in rock, soil, and water.
Uranium decays gradually (slowly) by emitting its alpha particle.
Uranium has poor electric conductivity (so poor conductor of electricity).
Uranium is malleable, ductile, and marginally paramagnetic
Occurrence of Uranium
Uranium is (naturally) found as uranium-238, uranium-235, and uranium-234.
The half-life of uranium-238 is about 4.47 billion years almost the age of the Earth and the half-life of uranium-235 is about 704 million years.
Alloys of Uranium
-
Following are the major alloys of Uranium −
Staballoy
Uranium hydride
Compounds of Uranium
-
Following are the major compounds of Uranium −
Uranium nitride - U2N3
Uranium pentafluoride - UF5
Uranium carbide - UC
Uranyl fluoride - UO2F2
Uranium dioxide - UO2
Uranium hexafluoride - UF6
Triuranium oxtoxide - U3O8
Uranium tetrafluoride - UF4
Uranium trioxide - UO3
Uranium tetrachloride - Ucl4
Uranyl nitrate - UO2(NO3)2
Uses of Uranium
Uranium is used as power source in nuclear submarines (especially by military).
Uranium is used in making nuclear weapons.
Uranium is also used as ballasts for ships.
Chemistry - Lead
Introduction
Lead is a heavy chemical element (metal) i.e. it has high density.
The symbol of lead is Pb and atomic number is 82.

Lead has tendency to bond itself; likewise, it can form chains, bonds, rings, and polyhedral structures.
Salient Features of Lead
Lead is soft and malleable metal; it has relatively low melting point.
Lead is relatively unreactive element and it has tendency to form covenant bond.
When lead is cut, it appears bluish-white tint.
While burning, lead gives a bluish-white flame (see the image given below).

Compounds of lead are typically found in the +2 oxidation state.
Occurrence of Lead
Lead is known to the prehistoric people of Western Asia.
Lead is found in the earths crust; it is rarely found deep of the earth.
Lead is usually found in combination with sulfur.
Galena is the main lead-bearing mineral, mostly found with zinc ores.
Alloys of Lead
-
Following are the major alloys of lead −
Molybdochalkos (copper)
Solder (tin)
Terne (tin)
Compounds of Lead
-
Following are the major compounds of lead −
Lead monoxide - PbO
Lead dioxide - PbO2
Uses of Lead
Lead has been used in making bullets for hundreds of years.
Lead is commonly used as a protective sheath for the underwater cables (only because it has the property of corrosion resistance).
Lead sheets are also used as architectural metals especially in roofing material.
Lead is also used in acid batteries.
Lead compounds are commonly as coloring agents and semiconductors.
Lead compounds are also used in plastic, candles, glass, etc.
Lead is commonly used in the polyvinyl chloride (i.e. used in coating of electrical cords).
Pre-caution
Presence of lead (in excessive quality) in the body may cause severe damage to the brain and kidneys; it may even cause death lastly.
Chemistry - Thorium
Introduction
Thorium is one of the radioactive actinide metals that occur naturally in large quantities.
The symbol of thorium is Th and atomic number is 90.

In 1829, a Norwegian mineralogist Morten Thrane Esmark, first discovered thorium.
Jns Jacob Berzelius, the Swedish chemist, identified and named it thorium after the name of Thor, the Norse god of thunder.
Salient Features of Thorium
Thorium is paramagnetic and soft radioactive actinide metal.
Thorium metals color is silvery; when it exposed to air, it tarnishes black and form dioxide.
All isotopes of thorium are unstable and it is a weak radioactive element.
Among all the significant radioactive elements, the half-life of thorium is the longest, i.e. about 14.05 billion years.
The melting point of thorium is about 17500C.
Occurrence of Thorium
Thorium is primordial element that exists existed in its current form since before the Earth was formed.
Thorium, found in the earths crust, is refined from the monazite sands.
Monazite that occurs in large amounts across the world is the most important source of thorium.
Alloys of Thorium
-
Mag-Thor and thorium-aluminum are the most significant alloys of thorium, Magnesium, and aluminum.
Compounds of Thorium
-
Following are the major compounds of Thorium −
Thorium dioxide - ThO2
Thorium (IV) sulfide - ThS2
Thorium (IV) iodide - ThI4
Thorium tetrafluoride - ThF4
Thorium (IV chloride - ThCl4
Thorium (IV) carbide - ThC
-
Some others are −
Thorite
Thorium (IV) nitrate
Thorium (IV) orthosilicate
Uses of Thorium
Thorium is normally used in gas tungsten arc welding (GTAW) because it (thorium) increases the high-temperature strength of tungsten electrodes and accordingly improve arc stability.
In electronic equipment, the application of thorium coating on tungsten wire, increases the electron emission of heated cathodes.
In chemical industry, the dioxide of thorium namely thoria is commonly used.
Chemistry - Hydrogen
Introduction
In the periodic table, hydrogen is the lightest element, its atomic weight is merely 1.008.
The symbol of hydrogen is H and the atomic number is 1.

In the early 16th century, hydrogen gas was first artificially produced by the reaction of acids and metals.
Henry Cavendish first recognized the hydrogen gas a discrete substance during the period of 1766-81, as it produces water when it is burned.
Salient Features of Hydrogen
In their plasma state, the non-remnant stars are primarily composed of hydrogen.
At standard temperature and pressure, hydrogen appears colorless, tasteless, odorless, nonmetallic, non-toxic, and highly combustible diatomic gas.
The molecular formula of hydrogen is H2.
On the earth, hydrogen exists in molecular forms, for example, water or other organic compounds.
Hydrogen also plays an important role in acidbase reactions.
Hydrogen gas is highly flammable in the air.
Pure hydrogen-oxygen flames radiate ultraviolet light; further, with high oxygen mix are nearly invisible to the naked eye.
Hydrogen can react with almost every oxidizing element.
At room temperature, Hydrogen normally reacts spontaneously and viciously with chlorine and fluorine and forms the corresponding hydrogen halides.
Occurrence of Hydrogen
Consisting roughly about 75 percent of all baryonic mass, hydrogen is the most abundantly found chemical subsistence in the universe.
Throughout the universe, hydrogen is typically found in the atomic and plasma states; however, the properties quite different from those of the molecular hydrogen.
On the earth, hydrogen exists as the diatomic gas, i.e. H2.
Because of having light weight, hydrogen easily escapes from the earths atmosphere.
Hydrogen is the third most abundant element found on the Earth's surface, but largely found in form of hydrocarbons and water.
Compounds of Hydrogen
-
Following are the major compounds of hydrogen −
Water - H2O
Ammonia - NH3
Hydrogen chloride - HCl
Hydrogen fluoride - HF
Hydrogen sulfide - H2S
Methane - CH4
Hydroxide - OH-
Hydrogen bromide - HBr
Hydrogen iodide - HI
Hydrogen cyanide - HCN
Phosphine - PH3
Hydrogen selenide - H2Se
Methanol - CH3OH
Lithium hydride - LiH
Bicarbonate - HCO3
Hydrogen telluride - H2Te
Liquid hydrogen - H2
Cyanide - CN
Calcium hydride - CaH2
Heavy water - D2O
Diborane - B2H6
Sodium hydride - NaH
Potassium hydride - KH
Uses of Hydrogen
The largest amount of H2 is used in the processing of fossil fuels as well as in the production of ammonia.
Hydrogen (H2) is extensively used in the petroleum and chemical industries.
H2 is typically used as a hydrogenating agent, especially in increasing the saturation level of unsaturated fats and oils.
H2 is also used as a shielding gas in welding procedures, such as atomic hydrogen welding, etc.
Chemistry - Helium
Introduction
Helium is the second lightest (after hydrogen) and second most abundant element in the universe.
The symbol of Helium is He and atomic number is 2.

In the periodic table, Helium is the first in the noble gas group.
Helium is named after the name of the Greek god of the Sun, Helios.
Salient Features of Helium
Helium is a colorless, odorless, tasteless, inert, non-toxic, and monatomic gas.
The boiling point (-268.90C) of helium is the lowest among all the elements.
Helium is typically composed of two electrons in atomic orbitals and surrounded by a nucleus, which consists two protons and two neutrons.
Occurrence of Helium
Most helium found in the universe belong to helium-4, and it is believed to have been formed during the Big Bang.
Major share of new helium is typically being created by nuclear fusion of hydrogen in stars including the Sun.
Though there is continuous creation of new helium; nevertheless, the availability of helium on the earth is substantially low because being the light weight element, it easily escapes into space.
In heterosphere (outer atmosphere) of the earth, helium is one of the most abundantly found elements (gases).
In the earths crust, helium is characteristically found in large amounts in the minerals of uranium and thorium.
Compounds of Helium
-
Following are the major compounds of helium −
Disodium helide - Na2He
Cristobalite He II (Silicates) - SiO2He
Dihelium arsenolite - As4O62He
Isotopes of Helium
-
There are about nine known isotopes of helium, but following two are the most stable isotopes −
Helium-3 and
Helium-4
Uses of Helium
Because of having low density, low boiling point, low solubility, high thermal conductivity, helium is widely used element; the most popular example is use of helium in balloon.
Major chunk of helium has cryogenic applications, such as, cooling the superconducting magnets used in medical MRI scanners and NMR spectrometers.
Helium is also used as a protective gas in growing silicon and germanium crystals.
Helium is also used in and gas chromatography and in titanium and zirconium production.
Helium is used in supersonic wind tunnels.
Helium is also applied as a shielding gas in an arc welding processes.
Chemistry - Oxygen
Introduction
Oxygen is the member of group 16 on the periodic table; however, most of the time, it is treated differently from its group.
The symbol of oxygen is O and atomic number is 8.
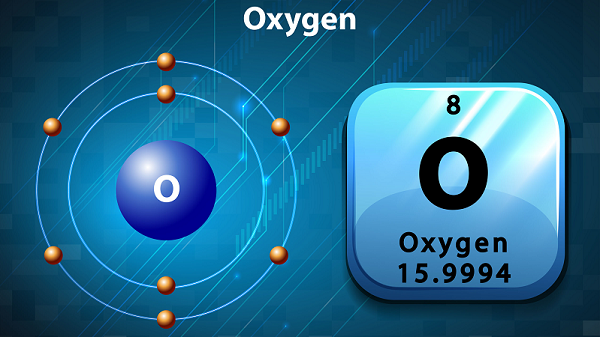
Oxygen has about nine allotropes and the most common allotrope is diatomic oxygen (i.e. O2). Other important allotrope is Ozone i.e. O3.
Oxygen, first time, was noticed by Swedish pharmacist Carl Wilhelm Scheele.
Salient Features of Oxygen
Oxygen is characteristically categorized as the member of chalcogen group.
The word "chalcogen" is derived from a Greek word khalks, which means copper and the Latin-Greek word Gens, which means born or produced.
Oxygen is a highly reactive gas (or nonmetallic element); hence, it is an oxidizing agent that readily forms oxides with most of the elements and compounds.
Oxygen has six valence electrons.
The melting point of oxygen is -218.80C and the boiling point is -1830C.
Occurrence of Oxygen
With about 20.8 percent share (in total earths atmospheric constituents), oxygen is the second ranked element of the earths atmosphere.
Oxygen occurs almost in sphere of the earth namely atmosphere, hydrosphere, and lithosphere.
During the photosynthesis process, free oxygen is produced by all green plants.
Oxygen occurs as constituent copper ores.
A human body contains about 65 percent oxygen.
By mass, almost half of the earths crust is composed of oxygen (i.e. its oxides).
By mass, oxygen is the third-most abundant element that found in the universe; the first and second are hydrogen and helium accordingly.
Oxygen (i.e. O2) is a colorless and odorless diatomic gas.
Oxygen dissolves in water very easily; however, the solubility of oxygen in the water is temperature-dependent.
Compounds of Oxygen
-
Following are the major compounds of oxygen −
Oxide
Peroxide
Carbon dioxide - CO2
Hydroxide - OH-
Ozone - O3
Mercury (II) oxide - HgO
Chlorate - ClO3
Aluminum oxide - Al2O3
Carbon monoxide - CO
Hypochlorite - ClO-
Silicon dioxide - SiO2
Hypofluorous acid - HOF
Sodium peroxide - Na2O2
Potassium chlorate - KClO3
Oxygen difluoride - OF2
Sodium oxide - Na2O
Uses of Oxygen
Oxygen (O2) is the most essential requirements for the respiration, without it, life cannot be imagined.
Oxygen is used in medicine.
Oxygen therapy is typically used to treat some diseases, such as, emphysema, pneumonia, some heart disorders, etc.
Some of the underwater activities, such as scuba diving, submarines, etc. also use artificial oxygen.

Aircrafts, mountaineers, etc. also use artificial oxygen.
Oxygen is also used in some of the industries, e.g. smelting of iron ore into steel in this process, about 55% of oxygen is used.
Chemistry - Carbon
Introduction
Carbon is a non-metallic and tetravalent element.
Tetravalent means carbon makes four electrons available to form the covalent chemical bonds.
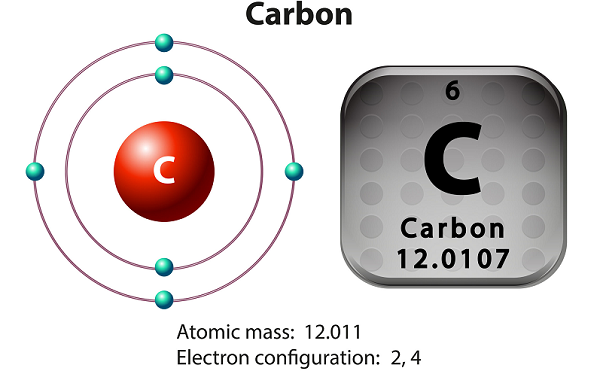
Carbon has three isotopes that occur naturally namely 12C, 13C, and 14C.
Among them, 12C and 13C are stable, but 14C is a radioactive isotope. Half-life of 14C is about 5,730 years.
Salient Features of Carbon
The physical properties of carbon largely depend on its allotropes.
Major allotropes of carbon are graphite, diamond, and amorphous carbon.
Graphite is opaque, black, and very soft; hence, it used to form a streak on the paper.
Diamond very hard (the hardest naturally occurring material) and transparent.
Graphite is a good conductor of electricity.
Diamond is bad conductor of electricity.
Carbon most likely has the highest sublimation point among all the elements.
Occurrence of Carbon
In terms of mass, carbon is the fourth most abundant chemical element found in the universe (after hydrogen, helium, and oxygen).
Carbon is available in abundance in the Sun, stars, comets, and in the atmospheres of most of the planets.
Carbon is found in the earths atmosphere and dissolved in water.
Hydrocarbons, such as coal, petroleum, and natural gas, all of them contain carbon.
Carbon is also found in methane hydrates, which found in polar regions and under the seas.
Some of the rocks enriched of carbon are coal, limestone, dolomite, etc.
Coal is very rich in carbon; hence, it is the largest commercial source of mineral carbon.
Coal shares about 4,000 gigatonnes or 80% of total fossil fuel.
Compounds of Carbon
-
Following are the major compounds of Carbon −
Cyanogen - CN2
Hydrogen cyanide - HCN
Cyanamide - CN2H2
Isocyanic acid - HNCO
Cyanogen chloride - CNCl
Chlorosulfonyl isocyanate - CNClO3S
Cyanuric chloride - NCCl3
Carbon disulfide - CS2
Carbonyl sulfide - OCS
Carbon monosulfide - CS
Uses of Carbon
Depending upon the allotrops, carbon is used in range of applications.
Carbon is one of the most essential elements of life without it, we cannot imagine life on the earth.
The fossil fuel namely methane gas and crude oil (petroleum), coal etc. are used in everyday life.
Graphite, combining with clay, used in making 'lead' used in pencils.
Charcoal is also used as a drawing material in artwork, iron smelting, barbecue grilling, etc.
Diamond is usually used in jewelry.
Industrial diamonds are used in cutting, drilling, and polishing tools for machining the metals and stone.
Fossil hydrocarbons, and carbon fiber are used in making plastic.
Chemistry - Nitrogen
Introduction
Nitrogen is a chemical element of group of 15 of the periodic table; among all the elements of group 15, it is the lightest element.
The symbol of nitrogen is N and atomic number is 7.

In 1772, Scottish physician Daniel Rutherford, first discovered and isolated carbon.
However, the name nitrogen was first given by Jean-Antoine-Claude Chaptal in 1790.
Salient Features of Nitrogen
Nitrogen has two stable isotopes namely 14N and 15N.
Free nitrogen atoms normally easily react with most of the elements and form nitrides.
The molecules of N2 is colorless, odorless, tasteless, and diamagnetic gas at standard conditions.
The melting point of N2 is 2100C and the boiling point is 1960C.
Nitrogen compounds repetitively interchange between the atmosphere and living organisms, making a nitrogen cycle.
Occurrence of Nitrogen
Nitrogen is most abundantly found element on the earth, as it constitutes about 78.1% of the entire volume of the earths atmosphere.
Nitrogen gas, which is an industrial gas, largely produced by the fractional distillation of liquid air.
Compounds of Nitrogen
-
Following are the major compounds of Nitrogen −
Ammonium - NH4+
Ammonia - NH3
Nitric acid - HNO3
Nitrite - NO2-
Nitrogen dioxide - NO2
Dinitrogen pentroxide - N2O5
Hydrazine - N2H4
Dinitrogen - N2
Cyanide - CN
Ammonium nitrate - (NH4)(NO3)
Nitrogen trichloride - NCl3
Nitrogen trifluoride - NF3
Nitrogen triiodide - NI3
Pyridine - C5H5N
Nitronium ion - NO2+
Hydrazoic acid - HN3
Ammonium sulfate - (NH4)2SO4
Uses of Nitrogen
Nitrogen compounds are extensively used in wide range of fields and industries.
Pure nitrogen is used as food additive.
Used in fire suppression systems especially for the information technology equipment.
Also used in manufacturing stainless steel.
Nitrogen is also used to inflate the tires of some of the aircraft and race cars.
Liquid nitrogen is used as a refrigerant.
Chemistry - Chemical Law
The laws of nature related to chemistry is known as chemical laws.
Chemical reactions, normally, are administrated by certain laws, which are observed and formulated in words become fundamental concepts in chemistry.
Following are the significant chemical laws −
| Laws | Explanation |
|---|---|
| Avogadro's Law | Equal volumes of all gases, at the same temperature and pressure, have the same number of molecules |
| BeerLambert law, (or simply Beer's law or LambertBeer law) | Explains the attenuation of light to the properties of the material through which it (light) passes |
| Boyle's Law | The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system |
| Charles' Law (also known as Law of Volume) | When the pressure on a sample of a dry gas is held constant, the Kelvin temperature and the volume will be directly related |
| Fick's Laws of Diffusion | Describes diffusion (of flux) |
| Gay-Lussac's Law | "All gases have the same mean thermal expansivity at constant pressure over the same range of temperature" |
| Le Chatelier's Principle ("The Equilibrium Law") | When any system at equilibrium is subjected to change in concentration, temperature, volume, or pressure, then the system readjusts itself to counteract (partially) the effect of the applied change and a new equilibrium is established |
| Henry's Law | The law calculates the concentration of gas in the solution under pressure |
| Hess's Law | The change of enthalpy in a chemical reaction (it means, the heat of reaction at constant pressure) is independent of the pathway between the initial and final states |
| Law of conservation of energy | Energy can neither be created nor be destroyed |
| Raoult's Law | The partial vapor pressure of each component of an ideal mixture of liquids is equal to the vapor pressure of the pure component multiplied by its mole fraction in the mixture |
| Faraday's Law Electrolysis | The amount of substance produced at an electrode is directly proportional to the quantity of electricity passed |
| Atomic Theory | Matter is composed of distinct units known as atoms |
| Khler Theory | Explains the process in which water vapor condenses and forms the liquid cloud drops |
| Van 't Hoff Equation | Describes change in the equilibrium constant of a chemical reaction |
| Transition State Theory | The reaction rates of elementary chemical reactions |
| GrotthussDraper Law | It describes that the light which is absorbed by a system/surface can bring a photochemical change |
| Kinetic Theory of Gases | Describes the behavior of a hypothetical ideal gas |
| Aufbau Principle | Explains that the electrons orbiting the atoms first fill the lowest energy levels and then second higher levels and so on and so forth |
| Hund's Rule | Explains that every orbital in a sublevel is singly occupied before any orbital is doubly occupied |
| Collision Theory | Based on the kinetic theory of gases, collision theory describes that the gas-phase chemical reactions occur when molecules collide with sufficient kinetic energy |
Chemistry - Discovery of Elements
Introduction
Most likely copper was the first element, which was mined and used by humans.
The evidence of earliest use of copper was found in Anatolia, which belongs to 6,000 BCE.
The lead was most likely the second element that humans start using.
The oldest known artifact of lead is statuette, which was found in a temple of Osiris, Abydos, Egypt.
The statuette of Osiris temple belongs to (about) 3,800 BCE.
The oldest known gold treasure was discovered in Varna, Necropolis (Bulgaria).
This gold treasure belongs to (about) 4,400 BCE.
Discovery of silver is almost same as of gold; its evidence was found in Asia Minor.
Some evidence say that the iron was known from (about) 5,000 BCE.
The oldest known iron objects, which was used by the humans, were found in Egypt (belongs to 4000 BCE).
The following table illustrates the significant elements with their discovery date and discovers −
| Element | Discoverer | Discovery Date |
|---|---|---|
| Copper | Middle East (Place) | About 9,000 BCE |
| Lead | Egypt (Place) | About 7,000 BCE |
| Gold | Bulgaria (Place) | About 6,000 BCE |
| Silver | Asia Minor (Place) | About 5,000 BCE |
| Iron | Egypt (Place) | About 5,000 BCE |
| Tin | About 3,500 BCE | |
| Sulfur | Chinese/India | About 2,000 BCE |
| Mercury | Egypt | 2,000 BCE |
| Phosphorus | H. Brand | 1669 |
| Cobalt | G. Brandt | 1735 |
| Platinum | A. de Ulloa | 1748 |
| Nickel | F. Cronstedt | 1751 |
| Bismuth | C.F. Geoffroy | 1753 |
| Magnesium | J. Black | 1755 |
| Hydrogen | H. Cavendish | 1766 |
| Oxygen | W. Scheele | 1771 |
| Nitrogen | D. Rutherford | 1772 |
| Barium | W. Scheele | 1772 |
| Chlorine | W. Scheele | 1774 |
| Manganese | W. Scheele | 1774 |
| Molybdenum | W. Scheele | 1781 |
| Tungsten | W. Scheele | 1781 |
| Zirconium | H. Klaproth | 1789 |
| Uranium | H. Klaproth | 1789 |
| Titanium | W. Gregor | 1791 |
| Chromium | N. Vauquelin | 1797 |
| Beryllium | N. Vauquelin | 1798 |
| Vanadium | M. del Ro | 1801 |
| Potassium | H. Davy | 1807 |
| Sodium | H. Davy | 1807 |
| Calcium | H. Davy | 1808 |
| Boron | L. Gay-Lussac and L.J. Thnard | 1808 |
| Fluorine | A. M. Ampre | 1810 |
| Iodine | B. Courtois | 1811 |
| Lithium | A. Arfwedson | 1817 |
| Cadmium | S. L Hermann, F. Stromeyer, and J.C.H. Roloff | 1817 |
| Selenium | J. Berzelius and G. Gahn | 1817 |
| Silicon | J. Berzelius | 1823 |
| Aluminium | H.C.rsted | 1825 |
| Bromine | J. Balard and C. Lwig | 1825 |
| Thorium | J. Berzelius | 1829 |
| Lanthanum | G. Mosander | 1838 |
| Rubidium | R. Bunsen and G. R. Kirchhoff | 1861 |
| Thallium | W. Crookes | 1861 |
| Indium | F. Reich and T. Richter | 1863 |
| Helium | P. Janssen and N. Lockyer | 1868 |
| Neon | W. Ramsay and W. Travers | 1898 |
| Xenon | W. Ramsay and W. Travers | 1898 |
| Fermium | A. Ghiorso et al | 1952 |
| Nobelium | E. D. Donets, V. A. Shchegolev and V. A. Ermakov | 1966 |
| Dubnium | A. Ghiorso, M. Nurmia, K. Eskola, J. Harris and P. Eskola | 1970 |
| Tennessine | Y. Oganessian et al | 2010 |
Chemistry - Elements With Their Valence
The following table illustrates significant elements and their valence −
| Element | Valence | Symbol | Atomic No. |
|---|---|---|---|
| Hydrogen | -1, +1 | H | 1 |
| Helium | 0 | He | 2 |
| Lithium | 1 | Li | 3 |
| Beryllium | 2 | Be | 4 |
| Boron | 3, 2, 1 | B | 5 |
| Carbon | -1, -2, -4, 4, 3, 2, 1, | C | 6 |
| Nitrogen | 0, -1, -2, -3,0, 5, 4, 3, 2, 1, | N | 7 |
| Oxygen | -1, -2, 0, 2, 1, | O | 8 |
| Fluorine | -1, 0 | F | 9 |
| Neon | 0 | Ne | 10 |
| Sodium | -1, 1 | Na | 11 |
| Magnesium | 2 | Mg | 12 |
| Aluminum | 3, 1 | Al | 13 |
| Silicon | -1, -2, -4, 4, 3, 2, 1 | Si | 14 |
| Phosphorus | -1, -2, -3, 0, 5, 4, 3, 2, 1 | P | 15 |
| Sulfur | -1, -2, 0, 6, 5, 4, 3, 2, 1 | S | 16 |
| Chlorine | -1, -2, 0, 6, 5, 4, 3, 2, 1 | Cl | 17 |
| Argon | 0 | Ar | 18 |
| Potassium | -1, 1 | K | 19 |
| Calcium | 2 | Ca | 20 |
| Scandium | 3, 2, 1 | Sc | 21 |
| Titanium | -1, -2, 0, 4, 3, 2, | Ti | 22 |
| Vanadium | -1, -2, 0, 5, 4, 3, 2, 1 | V | 23 |
| Chromium | -1, -2, -3, -4, 0, 6, 5, 4, 3, 2, 1 | Cr | 24 |
| Manganese | -1, -2, -3, 0, 7, 6, 5, 4, 3, 2, 1 | Mn | 25 |
| Iron | -1, -2, 0, 6, 5, 4, 3, 2, 1 | Fe | 26 |
| Cobalt | -1, 0, 5, 4, 3, 2, 1 | Co | 27 |
| Nickel | -1, 0, 6, 4, 3, 2, 1 | Ni | 28 |
| Copper | 4, 3, 2, 1, 0 | Cu | 29 |
| Zinc | 2, 1, 0 | Zn | 30 |
| Gallium | 3, 2, 1 | Ga | 31 |
| Germanium | 4, 3, 2, 1 | Ge | 32 |
| Arsenic | -3, 5, 3, 2, | As | 33 |
| Selenium | -2, 6, 4, 2, 1 | Se | 34 |
| Bromine | -1, 0, 7, 5, 4, 3, 1 | Br | 35 |
| Krypton | 2, 0 | Kr | 36 |
| Rubidium | -1, 1 | Rb | 37 |
| Strontium | 2 | Sr | 38 |
| Yttrium | 3, 2 | Y | 39 |
| Zirconium | 0, -2, 4, 3, 2, 1 | Zr | 40 |
| Niobium | -1, -3, 0, 5, 4, 3, 2, 1 | Nb | 41 |
| Molybdenum | -1, -2, 0, 6, 5, 4, 3, 2, 1 | Mo | 42 |
| Technetium | -1, -3, 0, 7, 6, 5, 4, 3, 2, 1 | Tc | 43 |
| Ruthenium | -2, 0, 8, 7, 6, 5, 4, 3, 2, 1 | Ru | 44 |
| Rhodium | -1, 0, 6, 5, 4, 3, 2, 1 | Rh | 45 |
| Palladium | 4, 2, 0 | Pd | 46 |
| Silver | 3, 2, 1, 0 | Ag | 47 |
| Cadmium | 2, 1 | Cd | 48 |
| Indium | 3, 2, 1 | In | 49 |
| Tin | -4, 4, 2 | Sn | 50 |
| Antimony | -3, 5, 3 | Sb | 51 |
| Tellurium | -2, 6, 5, 4, 2, 1 | Te | 52 |
| Iodine | -1, 0, 7, 5, 3, 1 | I | 53 |
| Xenon | 8, 6, 4, 3, 2, 0 | Xe | 54 |
| Cesium | -1, 1 | Cs | 55 |
| Barium | 2 | Ba | 56 |
| Lanthanum | 3, 2 | La | 57 |
| Cerium | 4, 3, 2 | Ce | 58 |
| Praseodymium | 4, 3, 2 | Pr | 59 |
| Neodymium | 4, 3, 2 | Nd | 60 |
| Promethium | 3 | Pm | 61 |
| Samarium | 3, 2 | Sm | 62 |
| Europium | 3, 2 | Eu | 63 |
| Gadolinium | 3, 2, 1 | Gd | 64 |
| Terbium | 4, 3, 1 | Tb | 65 |
| Dysprosium | 4, 3, 2 | Dy | 66 |
| Holmium | 3, 2 | Ho | 67 |
| Erbium | 3 | Er | 68 |
| Thulium | 3, 2 | Tm | 69 |
| Ytterbium | 3, 2 | Yb | 70 |
| Lutetium | 3 | Lu | 71 |
| Hafnium | 4, 3, 2, 1 | Hf | 72 |
| Tantalum | -1, -3, 5, 4, 3, 2, 1 | Ta | 73 |
| Tungsten | -1, -2, -4, 0, 6, 5, 4, 3, 2, 1 | W | 74 |
| Rhenium | -1, -3, 0, 7, 6, 5, 4, 3, 2, 1 | Re | 75 |
| Osmium | -2, 0, 8, 7, 6, 5, 4, 3, 2, 1 | Os | 76 |
| Iridium | -1, 0, 6, 5, 4, 3, 2, 1 | Ir | 77 |
| Platinum | 6, 5, 4, 2, 0 | Pt | 78 |
| Gold | -1, 0, 7, 5, 3, 2, 1 | Au | 79 |
| Mercury | 2, 1 | Hg | 80 |
| Thallium | 3, 1 | Tl | 81 |
| Lead | 4, 2 | Pb | 82 |
| Bismuth | -3, 5, 3, 1 | Bi | 83 |
| Polonium | -2, 6, 4, 2 | Po | 84 |
| Astatine | -1, 7, 5, 3, 1 | At | 85 |
| Radon | 2, 0 | Rn | 86 |
| Francium | 1 | Fr | 87 |
| Radium | 2 | Ra | 88 |
| Actinium | 3 | Ac | 89 |
| Thorium | 4, 3, 2 | Th | 90 |
| Protactinium | 5, 4, 3 | Pa | 91 |
| Uranium | 6, 5, 4, 3, 2 | U | 92 |
| Neptunium | 7, 6, 5, 4, 3, 2 | Np | 93 |
| Plutonium | 7, 6, 5, 4, 3, 2 | Pu | 94 |
| Americium | 7, 6, 5, 4, 3, 2 | Am | 95 |
Elements With Their Atomic Number
Atomic number defines the number of protons found in nucleus of an element.
The total number of protons and neutrons (found in nucleus) is calculated as the atomic mass number.
The following table illustrates the some of the significant elements with their atomic number, atomic mass, and symbols −
| Element | Atomic Number | Atomic Mass (g mol-1) | Symbol |
|---|---|---|---|
| Hydrogen | 1 | 1.0079 | H |
| Helium | 2 | 4.00 | He |
| Lithium | 3 | 6.94 | Li |
| Beryllium | 4 | 9.01 | Be |
| Boron | 5 | 10.81 | B |
| Carbon | 6 | 12.01 | C |
| Nitrogen | 7 | 14.0067 | N |
| Oxygen | 8 | 16.00 | O |
| Fluorine | 9 | 19.00 | F |
| Neon | 10 | 20.1797 | Ne |
| Sodium | 11 | 22.989768 | Na |
| Magnesium | 12 | 24.3050 | Mg |
| Aluminum | 13 | 26.981539 | Al |
| Silicon | 14 | 28.0855 | Si |
| Phosphorus | 15 | 30.973762 | P |
| Sulfur | 16 | 32.066 | S |
| Chlorine | 17 | 35.4527 | Cl |
| Argon | 18 | 39.948 | Ar |
| Potassium | 19 | 39.0983 | K |
| Calcium | 20 | 40.078 | Ca |
| Scandlum | 21 | 44.955910 | Sc |
| Titanium | 22 | 47.867 | Ti |
| Vanadium | 23 | 50.9415 | V |
| Chromium | 24 | 51.9961 | Cr |
| Manganese | 25 | 54.93805 | Mn |
| Iron | 26 | 55.845 | Fe |
| Cobalt | 27 | 58.93320 | Co |
| Nickel | 28 | 58.6934 | Ni |
| Copper | 29 | 63.546 | Cu |
| Zinc | 30 | 65.39 | Zn |
| Gallium | 31 | 69.723 | Ga |
| Germanium | 32 | 72.61 | Ge |
| Arsenic | 33 | 74.92159 | As |
| Selenium | 34 | 78.96 | Se |
| Bromine | 35 | 79.904 | Br |
| Krypton | 36 | 83.80 | Kr |
| Rubidium | 37 | 85.4678 | Rb |
| Strontium | 38 | 87.62 | Sr |
| Yttrium | 39 | 88.90585 | Y |
| Zirconium | 40 | 91.224 | Zr |
| Niobium | 41 | 92.90638 | Nb |
| Molybdenum | 42 | 95.94 | Mo |
| Technetium | 43 | 97.9072 | Te |
| Ruthenium | 44 | 101.07 | Ru |
| Rhodium | 45 | 102.90550 | Rh |
| Palladium | 46 | 106.42 | Pd |
| Silver | 47 | 107.8682 | Ag |
| Cadmium | 48 | 112.411 | Cd |
| Indium | 49 | 114.818 | In |
| Tin | 50 | 118.710 | Sn |
| Antimony | 51 | 121.760 | Sb |
| Tellurium | 52 | 127.60 | Te |
| Iodine | 53 | 126.90447 | I |
| Xenon | 54 | 131.29 | Xe |
| Cesium | 55 | 132.90543 | Cs |
| Barium | 56 | 137.327 | Ba |
| Lanthanum | 57 | 138.9055 | La |
| Cerium | 58 | 140.115 | Ce |
| Praseodymium | 59 | 140.90765 | Pr |
| Neodymium | 60 | 144.24 | Nd |
| Promethium | 61 | 144.9127 | Pm |
| Samarium | 62 | 150.36 | Sm |
| Europium | 63 | 151.965 | Eu |
| Gadolinium | 64 | 157.25 | Gd |
| Terbium | 65 | 158.92534 | Tb |
| Dysprosium | 66 | 162.50 | Dy |
| Holmium | 67 | 164.93032 | Ho |
| Erbium | 68 | 167.26 | Er |
| Thulium | 69 | 168.93421 | Tm |
| Ytterbium | 70 | 173.04 | Yb |
| Lutetium | 71 | 174.967 | Lu |
| Hafnium | 72 | 178.49 | Hf |
| Tantalum | 73 | 180.9479 | Ta |
| Tungsten | 74 | 183.84 | W |
| Rhenium | 75 | 186.207 | Re |
| Osmium | 76 | 190.23 | Os |
| Iridium | 77 | 192.217 | Ir |
| Platinum | 78 | 195.08 | Pt |
| Gold | 79 | 196.96654 | Au |
| Mercury | 80 | 200.59 | Hg |
| Thallium | 81 | 204.3833 | Tl |
| Lead | 82 | 207.2 | Pb |
| Bismuth | 83 | 208.98037 | Bi |
| Polonium | 84 | 208.9824 | Po |
| Astatine | 85 | 209.9871 | At |
| Radon | 86 | 222.0176 | Rn |
| Francium | 87 | 223.0197 | Fr |
| Radium | 88 | 226.0254 | Ra |
| Actinium | 89 | 227.0278 | Ac |
| Thorium | 90 | 232.0381 | Th |
| Protactinium | 91 | 231.0388 | Pa |
| Uranium | 92 | 238.0289 | U |
| Neptunium | 93 | 237.0482 | Np |
| Plutonium | 94 | 244.0642 | Pu |
| Americium | 95 | 243.0614 | Am |
| Curium | 96 | 247.0703 | Cm |
| Berkelium | 97 | 247.0703 | Bk |
| Californium | 98 | 251.0796 | Cf |
| Einsteinium | 99 | 252.083 | Es |
| Fermium | 100 | 257.0951 | Fm |
| Mendelevium | 101 | 258.10 | Md |
| Nobelium | 102 | 259.1009 | No |
| Lawrencium | 103 | 262.11 | Lr |
| Unnilquadium | 104 | 261.11 | Unq |
| Unnilpentium | 105 | 262.114 | Unp |
| Unnilhexium | 106 | 263.118* | Unh |
| Unnilseptium | 107 | 262.12 | Uns |
Chemistry - Nobel Prize
Jacobus Henricus van 't Hoff (a scientist of the Netherlands) was the first person who received the Nobel Prize in Chemistry in 1901.
Jacobus Henricus received the Nobel award for his work namely the laws of chemical dynamics and osmotic pressure in solutions.
Starting from the 1901 to 2016, total 174 scientists (of chemistry) have been received the Nobel Prize.
By the time, four women have been received the Nobel Prize in chemistry.
Marie Curie was the first lady who received the Nobel Prize in chemistry.
The following table illustrates the name of individuals who received Nobel Prize in chemistry along with their work (for which they received the Prize) −
| Name | Country (year) | Work/Area |
|---|---|---|
| Svante August Arrhenius | Sweden (1903) | Electrolytic theory of dissociation |
| Sir William Ramsay | UK (1904) | Discovery of the inert gaseous elements in air |
| Ernest Rutherford | UK/New Zealand (1908) | Chemistry of radioactive substances |
| Maria Skodowska-Curie | Poland/France (1911) | Discovery of the elements radium and polonium |
| Alfred Werner | Switzerland (1913) | Linkage of atoms in molecules |
| Theodore William Richards | US (1914) | Determinations of the atomic weight |
| Walter Norman Haworth | UK (1937) | Investigations on carbohydrates and vitamin C |
| Paul Karrer | Switzerland (1937) | investigations on carotenoids, flavins and vitamins A and B2 |
| Adolf Friedrich Johann Butenandt | Germany (1939) | Work on sex hormones |
| Otto Hahn | Germany (1944) | Discovery of the fission of heavy nuclei |
| John Howard Northrop & Wendell Meredith Stanley | US (1946) | Preparation of enzymes and virus proteins in a pure form |
| Vincent du Vigneaud | US (1955) | First synthesis of a polypeptide hormone |
| Sir Cyril Norman Hinshelwood & Nikolay Nikolaevich Semenov | UK & Soviet Union (1956) | Mechanism of chemical reactions |
| Frederick Sanger | UK (1958) | The structure of proteins (especially insulin) |
| Willard Frank Libby | US (1960) | Method to use carbon-14 for age determination |
| Melvin Calvin | US (1961) | Carbon dioxide assimilation in plants |
| Karl Ziegler & Giulio Natta | Germany & Italy (1963) | Chemistry and technology of high polymers |
| Dorothy Crowfoot Hodgkin | UK (1964) | Determinations by X-ray techniques |
| Paul J. Flory | US (1974) | Physical chemistry of macromolecules |
| Paul Berg | US (1980) | recombinant-DNA |
| Aaron Klug | UK (1982) | Development of crystallographic electron microscopy |
| Henry Taube | US (1983) | Mechanisms of electron transfer reactions |
| Robert Bruce Merrifield | US (1984) | Methodology for chemical synthesis on a solid matrix |
| Elias James Corey | US (1990) | Methodology of organic synthesis |
| Richard R. Ernst | Switzerland (1991) | Methodology of high resolution nuclear magnetic resonance (NMR) spectroscopy |
| Kary B. Mullis | US (1993) | Polymerase chain reaction (PCR) method |
| George A. Olah | US & Hungary (1994) | Carbocation chemistry |
| Peter Agre | US (2003) | Discovery of water channels (cell membranes) |
| Roger D. Kornberg | US (2006) | Molecular basis of eukaryotic transcription |
| Gerhard Ertl | Germany (2007) | Chemical processes on solid surfaces |
| Venkatraman Ramakrishnan, Thomas A. Steitz, & Ada E. Yonath | 2009 | Structure and function of the ribosome |
| Tomas Lindahl, Paul L. Modrich, & Aziz Sancar | 2015 | DNA repair |
| Jean-Pierre Sauvage, Fraser Stoddart, & Ben Feringa | 2016 | Design and synthesis of molecular machines |